Design data of the water chiller. In this article, we will look at the design data of a water-cooled centrifugal chiller in great detail. This is a pretty advanced cooldown video, so if you're new to the subject, I recommend starting with the basics.
Scroll down for Chiller Design Data's YouTube video tutorial.
I want to emphasize that this is design data only. Every cooler is different and you should check with your manufacturer for the relevant information. Results will vary from the real world and also with load.
 |
| Main-parts-of-a-chiller jcool |
In the illustration above, we show the main components of the cooler. The compressor, which is the driving force for the refrigerant around the system. The condenser that removes unwanted heat from the system and sends it to the cooling tower. The expansion
valve that expands the refrigerant and controls superheat in the compressor and the evaporator
 |
| Charts-chiller jcool |
Let's look at all the points on these two graphs to see the pressure, temperature, enthalpy, and entropy around this system. The graph on the left is our graph of temperature entropy v and the graph on the right is our graph of pressure enthalpy v.
· Point 1 is just before the compressor and evaporator outlet. It will be saturated/slightly superheated steam at low pressure and low temperature.
· Point 2 is just after the compressor, before the condenser. It is superheated steam at high pressure and high temperature.
· Point 3 is just after the condenser, but before the expansion valve. It will be a saturated liquid at high pressure and medium temperature.
· Point 4 is just after the expansion valve but before the evaporator. It will be low pressure, low temperature, and will be a mixture of liquid and vapor.
 |
| Water cooled chiller design data jcool |
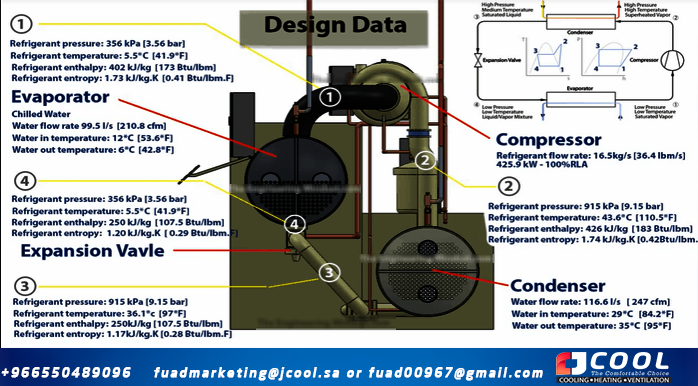
Compressor
In this example, the compressor drives a refrigerant at a flow rate of 16.5 kg/s (36.4 lbm/s). This motor then consumes 425.9 kilowatts and the compressor runs at 100% load. If the chiller is running at part load, the values will be different.
Refrigerant is withdrawn from the evaporator (point 1) at approximately 356 kPa (3.56 bar) and a temperature of 5.5 °C (41.9 °F). The enthalpy of the refrigerant is 402 kJ/kg (173 BTU/lbm). The entropy will be 1.73 kJ/kg.K (0.41 BTU/lbm.F).
The compressor compacts the refrigerant into a smaller space, and looking at our graphs, we know that the enthalpy will increase, the entropy will increase slightly, and the pressure and temperature will increase enormously.
At the coolant outlet (Point 2), it will be 915 kPa (9.15 bar). The temperature reached 43.6°C (110.5°F). The enthalpy is now 426 kJ/kg.K (183 Btu/lbm) and the entropy is now 1.74 kJ/kg.K (0.042 Btu/lbm.F).
Remember that the temperature of the refrigerant entering the condenser must be higher than the temperature of the water entering the condenser for heat transfer to occur. If they were the same temperature, there would be no heat transfer and the chiller would not cool.
Condenser
The next part we are going to look at is the capacitor. In this example, condenser water flows through the condenser at 116.6 l/s (247 cfm). Condenser water enters the condenser, from the cooling tower, at 29 °C (84.2 °F). The refrigerant will then transfer the unwanted heat from the building to the condenser water. This will raise the temperature of the condenser water so that when it returns to the cooling tower it will be around 95°F (35°C).
Now the reason the flux is higher in the condenser than in the evaporator is that the condenser has to reject more heat. You also need to remove heat from the compressor and other parts of the machine.
The refrigerant left the compressor and entered the condenser at a pressure of 915 kPa (9.15 Bar), a temperature of 43.6 °C (110.5 °F) with an enthalpy of 426 kJ/kg.K (183 Btu/lbm) and an entropy of of 1.74 kJ/kg.K (0.428 Btu/lbm.F).
Once the refrigerant has given up some of its energy to the circulating condenser water, it will now exit as a liquid at 36.1°C (97°F), but still at the same pressure that it entered. Its entropy will have dropped to 1.17 kJ.kg.K (0.28 BTU/lbm.F) and the enthalpy will rise to 250 kJ/kg.K (107.5 BTU/lbm). Then enter the regulator.
Expansion valve
The expansion valve controls the flow of refrigerant, measures superheat in the chiller suction line, and then reacts by allowing or limiting refrigerant flow to maintain a certain value. The refrigerant enters the expansion valve in liquid form and leaves in the form of a vapor/liquid mixture.
In this example, a temperature of 36.1°C (97°F) is entered, a pressure of 915 kPa (9.15 Bar), the entropy is 1.17 kJ.kg.K (0.28 BTU/lbm.F) and the enthalpy is 250 kJ/kg.K (107.5 BTU/lbm).
The coolant expands through a small hole that sprays the coolant. It expands into a larger volume and its pressure drops accordingly, allowing it to cool down because it's not as tight anymore. It will come out at a temperature of 5.5°C (41.9°F), a pressure of 356 kPa (3.56 Bar) and from the diagrams we know that it will maintain the same enthalpy but the entropy will change slightly and it will come out at 1.20 kJ / kg.K (0.29 BTU/lbm.F).
Evaporator
The evaporator generates cold “chilled water” that circulates through the building, providing air conditioning and collecting unwanted heat from the building. This now hot chilled water returns to the evaporator and transfers this heat to the chiller, the chilled water then leaves the chiller and surrounds the building, while the chiller boils and carries the heat energy to the compressor.
In this example, cold water is flowing through the evaporator at about 99.5 liters per second, or about 210 cubic feet per minute. Chilled water enters the evaporator at approximately 12°C (53.6°F). After the chilled water has transferred its heat to the condenser, it leaves the evaporator at approximately 6 °C (42.8 °F).
The coolant picks up heat energy but the temperature only changes slightly, which confuses many people. The reason it doesn't increase dramatically is because it undergoes a phase change from liquid to vapor, so heat energy is used to break the bonds between molecules. Enthalpy and entropy will increase and that's where the energy goes.
At the outlet of the refrigerant there will be a slightly superheated vapor at 5.5°C (41.9°F), a pressure of 356 kPa (3.56 Bar) and an entropy of 402 kJ/kg.K (173 Btu/lbm). and an enthalpy of 1.73 kJ/kg.K (0.41 btu/lbm.F).
The refrigerant then returns to the compressor to start the cycle again.
Do you need help maintaining
and repairing an air conditioner?
It's hard to keep cool when the air conditioning
isn't working. Whether it's repairs, air conditioning,
regular maintenance, or assistance with choosing
your new unit, JCOOL professionals can keep
you comfortable all year
Jamjoom Cooling Systems Factory (JCOOL)
products (condenser coil - evaporator coil -
heat exchanger- air conditioning -
cold evaporator - cooler -industrial air cooler
- tube bundle - air heat exchanger)
Make a reservation immediately with the
maintenance teambefore the summer heat
intensifies.
Let us help you with a lot of maintenance
and installation work on your next project.
To request the service: -
Jamjoom Cooling Systems Factory
Jeddah - Second Industrial City - Street 49
fuadmarketing@jamjoomarcool.com
fuadmarketing@jcool.sa
Fuad00967@gmail.com
Eng/ Abu Hussam
#heatExchangers #condensers #evaporators
#coolers #coils #airDucts #chiller's #jcool
#Saudi_industry #cooling #ventilation
#radiators #jcool #jamjoomCoil #jamjoom_cooling_systems_factory
#jamjoom #saudiArabai #coolingtowers #cooling_tower #coolingcoils
#heat_exchanger #heatexchanger #coolingsystems #cooling #chiller
#hvac #jamjoom_hvac #jamjoom_cooling #global_cooling_tower
#Brand_Saudi_Arabia #made_in_Saudi_Arabia #🇸🇦



Comments
Post a Comment